Navigation
Evolutionary timeline determined for malaria parasites in mammals, birds, reptiles
Scientists have determined the evolutionary timeline for the microscopic parasites that cause one of the world's most widespread infectious diseases: malaria.
|
Scientists have determined the evolutionary timeline for the microscopic parasites that cause one of the world's most widespread infectious diseases: malaria.
Having an understanding of the origins of the lineages of such pathogens, or disease-causing organisms, is fundamental to understanding emerging diseases, according to the researchers.
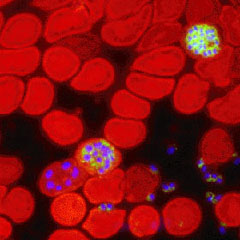
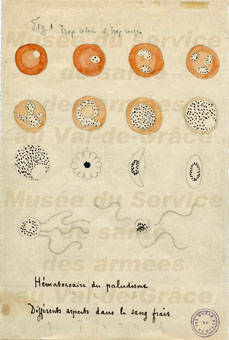 |
| An 1800s sketch by Alphonse Laveran, the French doctor who identified the malaria parasite. Credit: U.S. Centers for Disease Control |
The origin of malaria in humans has been dated to as recent as 10,000 years ago and as long as several million years ago.
Now biologists Robert Ricklefs of the University of Missouri-St. Louis and Diana Outlaw of Mississippi State University in Starkville have found a molecular clock for malaria parasites that provides a more precise date.
The results of their research, funded by the National Science Foundation (NSF), appear in last week's issue of the journal Science.
The findings provide a well-supported time calibration for the evolution of malaria parasites.
By marrying DNA research with a new statistical approach, the biologists were able to get a better handle on the timeline of parasite evolution.
The scientists found that a key gene in malaria parasites evolved at 60 percent of the rate of the same gene in its hosts.
Knowing the rate of gene evolution of the vertebrate hosts, the biologists were able to estimate that modern malaria parasites began to diversify across mammals, birds and reptiles about 16 million years ago.
The ancestors of humans acquired the parasite 2.5 million years ago.
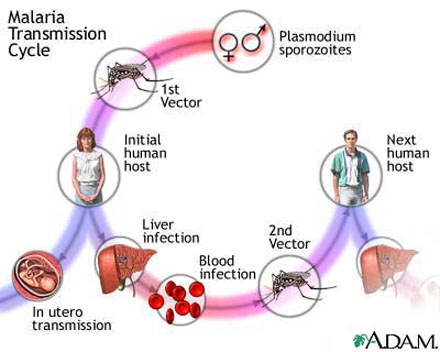 |
| Malaria in humans starts with the bite of a mosquito infected with a malaria-carrying parasite. Credit: National Institutes of Health |
"Malaria parasites undoubtedly were relatively benign for most of that history, becoming a major disease only after the origins of agriculture and dense human populations," said Ricklefs.
"These findings are important in providing a quantitative rate of evolution for malaria," said Alan Tessier, program director in NSF's Division of Environmental Biology, which funded the research.
"They also reveal that host-switching can result in a rapid diversification of parasites, and decouple their evolution from that of their hosts," Tessier said.
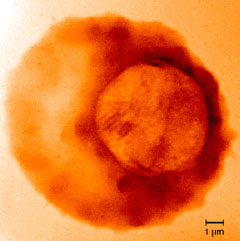 |
| Malaria parasite within a human red blood cell: where the disease begins. Credit: Lawrence Berkeley National Laboratory |
"Because single-celled malaria parasites leave no fossil record, one has to estimate their rate of evolution by comparison with their hosts," said Ricklefs.
"Previously, this had been done under the assumption that parasites evolve at the same rate as their hosts and thus were the same age as their hosts."
Ricklefs and Outlaw's research suggests that the parasites may jump to new, unrelated hosts at any time.
"One cannot equate parasite evolution," said Ricklefs, "with a host's evolution."
This article is from the National Science Foundation (NSF), July 8, 2010.
Search
Latest articles
Agriculture
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Air Pollution
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Biodiversity
- It is time for international mobilization against climate change
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Desertification
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- UN Food Systems Summit Receives Over 1,200 Ideas to Help Meet Sustainable Development Goals
Endangered Species
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
- Coral Research in Palau offers a “Glimmer of Hope”
Energy
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Wildlife Preservation in Southeast Nova Scotia
Exhibits
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Coral Reefs
Forests
- NASA Satellites Reveal Major Shifts in Global Freshwater Updated June 2020
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Global Climate Change
- It is time for international mobilization against climate change
- It is time for international mobilization against climate change
Global Health
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- More than 400 schoolgirls, family and teachers rescued from Afghanistan by small coalition
Industry
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Natural Disaster Relief
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
News and Special Reports
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
Oceans, Coral Reefs
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Pollution
- Zakaria Ouedraogo of Burkina Faso Produces Film “Nzoue Fiyen: Water Not Drinkable”
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Population
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Public Health
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Rivers
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Toxic Chemicals
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Actions to Prevent Polluted Drinking Water in the United States
Transportation
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Urbanization Provides Opportunities for Transition to a Green Economy, Says New Report
Waste Management
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water and Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution