Navigation
Development of Alternatives to Animal Use for Safety Testing and Hazard Assessment
Development of testing methodologies which are both more predictive of human response to a broad range of chemicals and finished products and less costly in animal lives and suffering.
Location:
United States of America
Problem Overview:
Development of testing methodologies which are both more predictive of human response to a broad range of chemicals and finished products and less costly in animal lives and suffering.
Since the passage of the Food, Drug and Cosmetic Act and other legislation meant to assure human health and safety via mandated testing of consumer products and chemicals, animals have been used as test subjects. Over the past sixty years, a broad range of animal tests has been developed to predict possible adverse effects of chemical ingredients and finished products. Some of the first tests to be developed and required by regulatory agencies in the U.S. and throughout the world included the Draize tests for eye and skin irritation and the LD50 test for acute toxicity. Other tests to assess carcinogenicity (ability to cause cancer), genotoxicity (ability to damage genetic material), reproductive toxicity (ability to harm the developing fetus or organs of reproduction), neurotoxicity (effect on the brain and nervous system), and other adverse effects swiftly followed. By 1975, a broad range of tests requiring large numbers of animals were recommended by the Organization for Economic Cooperation and Development (OECD), whose 29 member countries produce two-thirds of the world’s good and services, to establish the safety of products.
Nonetheless, developments within science were causing some to question the validity and usefulness of some animal tests. For example, in 1976, Swiss toxicologist Gerhard Zbinden, then director of the Institute of Toxicology at the University of Zurich, charged that the LD50, a test used worldwide to establish acute toxicity by determining the amount of a substance required to kill half a population of fifty to two hundred test animals, was “a wasteful endeavor in which scientific inventiveness and common sense have been replaced by a thoughtless completion of senseless protocols.” A few years later, Zbinden further alleged that the clinical experience indicated that the LD50 value in animals “rarely bears a meaningful relation with the lethal dose in man.”
In an 1989 article titled “Environmental Pollution and Cancer: Some Misconceptions” Bruce N. Ames, director of Environmental Health Sciences at the University of California, Berkeley and Lois Swirsky Gold, staff scientist at the Lawrence Berkeley Laboratory and director of the Carcinogenic Potency Project, stated unequivocally that “animal cancer tests conducted at near-toxic doses of the test chemical cannot predict the cancer risk to humans at the much lower doses to which they are typically exposed. The prediction of cancer risk requires knowledge of the mechanisms of carcinogenesis...recent understanding of these mechanisms undermines many of the assumptions of current regulatory policy regarding rodent carcinogens and requires a reevaluation of the purpose of routine animal cancer tests.”
The same problem was evident in animal tests for teratogenicity, Ames and Gold indicated, when test animals were fed doses of chemicals which far exceeded the amount of a chemical to which humans were naturally exposed. “Alcohol is the most important known human chemical teratogen. In contrast, there is no persuasive evidence that TCDD (dioxin) is either carcinogenic or teratogenic in humans, although it is both in rodents at near toxic doses. If one compares the potential of TCDD for causing birth defects with that of alcohol (after adjusting for their relative potency as determined in rodent tests) then daily consumption of TCDD at the EPA reference dose (the allowable dose) would be equivalent in teratogenic potential to a daily consumption of alcohol from 1/300,000 of a beer. That is equivalent to drinking a single beer (15 g ethyl alcohol) over a period of 8,000 years.”
At the same time that some scientists were instituting a critique of commonly performed protocols and their impact on regulatory policy, a social movement interrogating the human use and treatment of animals began to assert moral and ethical objections to the use of animals for product safety testing. This movement, although apparently appearing de novo in the middle decades of the twentieth century, nonetheless expressed longstanding objections to the scientific study of animal physiology through vivisection (French, 1975; Lansbury, 1985; Orlans, 1993; Rupke, 1990; Vyvan, 1969). By 1971, the number of animals used in scientific research and testing throughout the world exceeded 100 million, with at least 51 million animals per year used in research and testing in the United States alone (Rowan, 1984). Rats and mice comprised the great majority of laboratory animals, but dogs, cats, rabbits, hamsters, guinea pigs, birds, frogs and primates were also commonly used as research subjects.
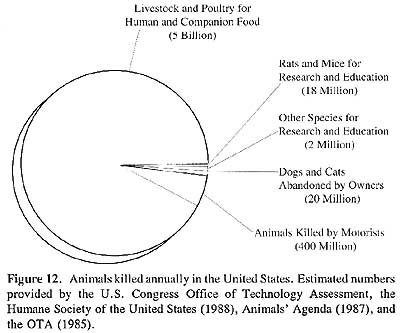 |
Within a relatively brief period of time in the early 1980’s, the animal rights movement was able to mobilize large numbers of Americans who objected to the practice of animal testing, particularly of cosmetics. Movement leaders were quick to seize upon cosmetics as a particularly effective tool to illustrate both the suffering of animals used as test subjects for eye and skin irritation, and the apparently trivial cause of their suffering--the development of lipsticks, skin creams, and other nonessential items. (Blum, 1994; Jasper & Nelkin, 1992; Rowan, Loew & Weer, 1995) For example, the Draize test for eye irritation was viewed by many animal advocates as a particularly egregious example of cruelty, although it was viewed as a scientific advance when developed in the nineteen forties. In the standard Draize test, a test substance was placed in one eye of a group of rabbits. The rabbits’ eyes were inspected periodically (usually at 24, 48 and 72 hours and at 4 and 7 days) after administration of the test substance and scientists observed and quantified changes in the cornea, conjunctiva and iris. Recovery from damage was also observed and measured. Animal protectionists argued that the test was both scientifically crude and cruel. Some scientists (Smyth, 1978; Weil and Scala, 1971) also supported replacement of the test, but in 1980 the great majority of toxicologists and regulatory officials maintained that the test, despite its acknowledged flaws, was both useful and necessary because it provided some basis for standardization and quantification of a product’s potential for irritation and more serious damage to the eye.
Although the limitations of available testing practices were increasingly evident, reformers were confronted with a dilemma. Although some scientists were willing to admit that available tests were inadequate, in 1978, few non-animal tests were available (notwithstanding Bruce Ames’ development of a bacterial test for mutagenicity in 1971). It was clear that the traditional protocols would never be replaced without a concerted effort to develop new tests. Such an endeavor would require large sums of money for research and a long-term commitment by key stakeholders to what would likely be a protracted and contentious transition from animal tests to “alternatives.” This has indeed been the case. The commitment of the cosmetics industry, fueled by consumer objections to animal testing, has created large-scale insitutional change. By 1999 an international effort to develop, validate and implement alternatives to animal use in other areas is also underway.
Background/History:
Animal protection
The modern animal rights movement is a product of two converging strains of modern thought regarding the role and status of animals and the human relationship to animals. The first, epitomized in the humane movement, sought to improve treatment of animals in a number of different areas without challenging the inherent right of humans to use animals as sources of food, labor, clothing and entertainment. The American Society for the Prevention of Cruelty to Animals (ASPCA), founded by Henry Bergh in 1866, was the first formal group established in the U.S. to work for better conditions for animals. While attempting to build support for the founding of the group, Bergh gave a lecture in New York City in which he noted that the prevention of animal cruelty “is a matter purely of conscience...it is a moral question in all its aspects; it addresses itself to that quality of our nature which cannot be disregarded by any people with safety to the dearest interests. It is a solemn recognition of that greatest attribute of the Almighty Ruler of the universe, mercy, which if suspended in our own case for a single instant, would overwhelm and destroy us.” (Niven, 1967)
Soon after founding the ASPCA, Bergh successfully lobbied for the passage of the nation’s first anticruelty law passed by the New York State legislature in April 1866. The work of the ASPCA was soon taken up by a number of other state and local humane societies which shared its commitment to improving the quality of lives of animals used by humans for a broad range of uses. Bergh and the humane societies stressed the important role that animals played in human society and the need for society to protect them. (Loeper, 1991;Turner, 1980)
A second strain of nineteenth century animal protection activity was far more radical than that championed by the ASPCA and other humane groups and focused exclusively on the use of animals as the subjects of scientific research. The antivivisection movement was adamantly opposed to such research and worked to abolish the use of animals in science. Bergh himself was an antivivisectionist, however after his death the ASPCA retreated from a pure antivivisectionist position and adopted a more moderate posture--seeking to limit abuses but in general accepting the need for animals to be used in science, as they were used for labor and food. The American Antivivisection Society, founded by Caroline Earle White in 1883, was the first formal group championing the antivivisection cause founded in the U.S. White had previously helped found the Pennsylvania Society for the Prevention of Cruelty to Animals in 1867. The founding of the AAVS was followed by the establishment of other state and local antivivisection societies throughout the United States. (Lederer, 1987 and 1995)
Although the humane movement was able to exert a certain degree of influence as an advocate for animals throughout the next hundred years, the antivivisection movement withered as scientists began to discover the causes, cure and prevention of various human diseases as a result of animal research. As the power of the scientific community grew, the influence of the antivivisectionists waned. However, by the 1970’s certain key events initiated a rebirth of the antivivisection movement under a new name--animal rights. Although the animal rights movement has a much broader agenda than simple antivivisection, its first targets were scientists and the use of animals for scientific research, and its first successes, which led to a massive growth in numbers and influence, were led by antivivisectionists.
The intellectual underpinnings of the modern animal rights movement have been provided by philosophers Peter Singer and Tom Regan. Singer, the author of Animal Liberation, published in 1975, is often called the father of the modern animal rights movement although his utilitarian philosophy does permit some limited use of animals. Tom Regan, author of The Case for Animal Rights, published in 1983, promotes a more pure philosophy of animal rights advocating the complete abolition of all forms of animal use. In this latter view, all human uses of animals are exploitative and oppressive in that they violate an animal’s right to exist free of human predation. It is important to note that even prior to the publication of Singer and Regan’s books, certain individuals and organizations, particularly in England, had already begun to operate according to animal rights principles, propounding a philosophy of animal “liberation” (Finsen & Finsen, 1998; Garner, 1993). However, it was not until the publication of these and other philosophical justifications, particularly Singer’s Animal Liberation, that the movement began to receive serious consideration and to recruit large numbers of adherents.
Toxicology and Safety Testing
Although human beings have long sought to evaluate the hazards of various substances, both natural and manmade, by first testing their effect on animal or human “tasters,” (including condemned prisoners) the establishment of a science of toxicology, or the systematic study of toxins, is a relatively recent development. Both pharmacology and toxicology were born as experimental sciences in the nineteenth century. It is interesting to note that both sciences were, from their inception, inextricably entwined with the growth of animal experimentation, and thus “from their very beginnings were confronted by opposition to vivisection experiments.” (Parascandola, 1991).
The first law in the United States mandating testing of consumer products was the Food, Drug and Cosmetic Act passed by Congress in 1938. This legislation was enacted after a series of accidents in which Americans were harmed by various drugs and cosmetics, including the death by poisoning of 107 Americans by a contaminated drug preparation called Elixir of Sulfanilimide, and severe eye injuries caused by a eyebrow/eyelash dye named Lash-Lure (Lamb, 1936). Although earlier legislation had prohibited interstate commerce of adulterated food, drink and drugs (Green and Bradlaw, 1992), they did not require safety testing or mandate that the manufacturer establish tolerance levels for these substances. John Draize, a pharmacologist who had previously researched the effects of agents used in chemical warfare, was hired by the FDA to develop eye and skin tests to determine the safety of cosmetic products.
“The FDC Act of 1938 established the concept that the cosmetic manufacturer must substantiate the safety of ingredients and finished products prior to marketing the product. Under the law, any ingredient or product whose safety is not adequately substantiated prior to marketing is inadequately labeled (i.e. misbranded) unless it is labeled with the statement ‘The safety of this product has not been determined,’ wrote James P. McCulley and Thomas J. Stephens in 1993. “Interestingly, the authors of the Act did not specify the types of tests required to substantiate the safety of products. This wording was not an accident. They undoubtedly expected the methods of testing to improve with time and did not want the law to specify outdated test methods.” (McCulley & Stephens, 1993).
Twenty years after the passage of the Food, Drug and Cosmetic Act, Congress passed the Delaney Amendments to the Act, which required that manufacturers furnish data proving that their products did not cause cancer. The Delaney amendments resulted in a much increased use of laboratory animals, particularly rodents, as manufacturers attempted to meet the requirements of the law. In 1976, Congress passed the Toxic Substances Control Act which gave the U.S. Environmental Protection Agency the authority to require testing of potentially harmful substances and the power to ban or restrict the manufacture or use of any chemical deemed hazardous. A number of other government regulatory agencies also either began to conduct testing themselves or to require tests of the products they were responsible for regulating. “As public pressures to protect the consumer heightened, additional legislation in the 1950’s and 1960’s introduced or strengthened regulations concerning the premarket testing of food additives, color additives, insecticides, drugs, and other products. At the same time, concerns about toxins in the environment and in the workplace led to further legislation concerning the testing and control of toxic substances. These developments contributed to a significant increase in toxicity testing, most of it involving the use of animals.” (Parascandola, 1991)
Traditional toxicology is based upon the assumption that physiological processes in mammals are much the same and that a substance which is toxic to a small mammal like a rodent is likely to prove equally toxic to a human being, if the quantity of the substance is adjusted according to body weight and other factors. Early tests mirror the concerns of the time. For example, in the wake of the cosmetics scare in the 1930’s, the Draize tests for eye and skin irritation were developed. The LD50, a test developed in 1927 to measure the potency of biological preparations such as vaccines was converted to use as a predictor of acute toxicity, establishing the lethal dose of a drug, chemical or other product. The LD50 assesses acute oral toxicity, and provides an estimate of the quantity of a substance which will kill half the members of a test population. Like the Draize test, the LD50 has been the focus of much criticism by both scientists and animal protectionists who object to exposing animals to high doses of toxic substances. This approach has been dubbed by critics the “kill ‘em and count ‘em” school of testing.Tests developed in the wake of the Delaney amendments, sought to establish the possible effects of continuous exposure to potential toxins (chronic toxicity) and a range of other biological effects, including effects on the reproductive, nervous and endocrine systems. Most of these tests also used large numbers of animals and generally used death or extreme morbidity as endpoints.
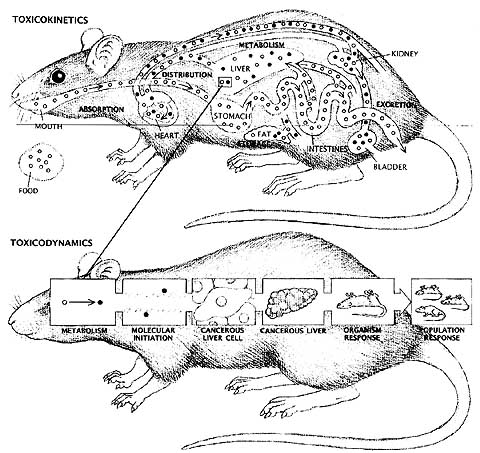 |
All of these are aspects of risk assessment, the process by which substances are evaluated for their potential impact on human health and safety. Risk assessment can be subdivided into two categories--assessment of exposure, which is an estimate of the number of people who may be exposed to a particular chemical, plus concentration, duration and terms of exposure--and toxicity testing, which identifies hazards. Toxicity testing is also expected to predict the type of adverse effects which may follow exposure to a chemical and provide an estimate of the exposure-response relationship. Many factors may affect the latter, including age, sex, genetic background, and health status of the subject.
The Process of Change
As the powerful science of molecular biology began to be applied in toxicological research, the flaws and limitations of some traditional protocols became apparent. Scientists sought to discover the mechanisms of action of various substances, seeking to understand how a toxin affected the body at the molecular level. Toxicokinetic studies traced the absorption, metabolism, storage and excretion of chemicals in the body. This type of study was carried out in vivo or in the living animal. Toxicodynamic studies attempted to define the biological consequences of chemical activity. Much of this research was undertaken in vitro (in glass) in cells or tissues taken from animals or human beings. Both types of studies began to reveal interesting differences in the manner in which various species metabolized certain chemicals, calling into question the basic assumption that responses observed in rodents were valid predictors of human toxicity. A few studies indicated that some animal tests were not as reliable as predictors of human response as was commonly assumed (Weil and Scala, 1971). Some scientists began to propose the adoption of a “parallelogram approach” to extrapolation for risk assessment. First advocated by Sobels in 1977, the parallelogram approach uses in vitro data to test the hypothesis that a specific mechanism of action is conserved among rodent and human species. As the questions posed (and answered) by molecular toxicology became ever more precise and sophisticated, the gap between those trained in the in vitro approach and in vivo traditionalists became more pronounced.
Despite increasing evidence indicating inefficacy and waste in many standard in vivo tests, such protocols were firmly entrenched as the basis of a complex international regulatory apparatus. In order to make changes to testing protocols recommended by the OECD, all 29 member nations must agree to alterations. Due to the need for consensus, rapid and dramatic changes in OECD policy and recommended protocols are unlikely to occur. Both within member nations and in the international community as represented by the OECD, bureaucratic inertia and the weight of tradition mitigated against any type of large scale critique and replacement of traditional methodologies. But in the late 1970’s, pressure exerted by the nascent animal rights movement created a groundswell of public protest which forced a re-evaluation of testing practices.
 |
| Henry Spira |
In 1976, Henry Spira, a New York City public school teacher with decades of experience in human rights activism, successfully completed a two year campaign against The American Museum of Natural History in New York City. Spira and his allies exerted sufficient pressure to persuade the National Institutes of Health to stop funding experiments at the Museum on the sexual behavior of cats deprived of various types of sensory functioning. The resulting public protest and dismantling of the Museum’s laboratories served as a stimulus to Spira’s next campaign to repeal the Metcalf-Hatch Act in New York State. This Act, passed in 1952, permitted researchers to use unclaimed dogs and cats from pounds throughout the state as research subjects. In 1979 Spira’s group was able to create a public and legislative dialogue which resulted in repeal of the Act.
The group’s next target was the cosmetic company Revlon. As in previous campaigns, the activists led by Spira contacted the company’s leadership and attempted to persuade them to re-evaluate their testing practices. When, after eighteen months of sustained letters, phone calls and personal interviews, this strategy failed, a coalition of animal protection groups led by Spira launched a public campaign. In April 1980, the coalition placed a full page ad in the New York Times protesting the company’s use of the Draize test for eye irritation. Public protest in the U.S. and Europe resulted in productive discussions between company leaders and activists. The result was a $250,000 grant by Revlon to Rockefeller University to fund research into alternatives to the Draize test. Soon after, Spira and colleagues approached Avon Products and that company, in conjunction with the cosmetics trade group CTFA (Cosmetics, Toiletry and Fragrance Association) donated $1 million to fund a more ambitious alternatives research program based at Johns Hopkins University.
 |
| Joanne Zurlo, Associate Director of the Johns Hopkins Center for Alternatives to Animal Testing |
This group, The Johns Hopkins Center for Alternatives to Animal Testing or CAAT, began sponsoring symposia and workshops to bring together researchers interested in pursuing alternatives research. The group also funded grants for scientists interested in pursuing this work and actively worked to bring together individuals with varying perspectives on the issue.
CAAT was not alone in adopting this strategy of dialogue and collaboration. Andrew N. Rowan, an Oxford educated biochemist who had previously worked as chief scientific officer for FRAME, a British group which champions an approach similar to that adopted by CAAT, played a major role in bringing together individuals and organizations promoting a wide range of views on the subject of animal testing (Rowan, 1987). Rowan, who served as science adviser to Henry Spira on the Revlon campaign, and
 |
| Alan Goldberg, Director of the Johns Hopkins Center for Alternatives to Animal Testing |
Alan Goldberg, director of CAAT, created an Animal Issues Discussion Group in the mid-80’s. Rowan, at that time Director of the Center for Animals and Public Policy at Tufts University, had already created a group composed of both animal protectionists and scientists to further dialogue and to identify common ground. Rowan and Goldberg created a second group, known as the Animal Issues Discussion Group. This corporate group attempted to understand both the ethical and practical implications of the animal rights movement and its effect on their companies.
The group met three times a year, with participation by approximately ten to fifteen high-ranking executives from major multi-national corporations. In most cases, attendees were designated “animal issues” point men and women, who reported directly to their CEO’s on the subject. Rowan and Goldberg brought in outside speakers, bioethicists, animal advocates, lawyers and others who made short presentations on the issue and then participated in question and answer sessions with attendees. The Rowan/Goldberg initiatives were the first attempts to bring together former adversaries on the testing issues in a neutral setting and to begin establishing possible ground for collaboration and cooperation.
The success of these efforts can perhaps be gauged by the fact that five years after the founding of CAAT, in 1985, the CTFA claimed a reduction of over 75% in the number of animals used in acute oral safety testing (LD50) and by the tenth anniversary of Hopkins center, a spokesman for the same group said that the use of animals for eye irritation testing in the United States had declined by 87% (Rudacille, 1992). At CAAT’s tenth anniversary symposium, its director Alan M. Goldberg, commented that “incredible progress has been realized by the coordinated efforts of scientists, educators, policymakers and animal welfarists who have forged a foundation over the past decade for the field of in vitro alternatives.” Henry Spira, who received a Founder’s Award at CAAT anniversary celebration, similarly commented in a 1985 article, “the current promising situation is the result of many individuals working together over a long period of time. Together and cooperatively we helped build a consensus among all parties that these wasteful tests are not needed.” (Spira, 1985)
Implementation:
Definition
The reform strategy of Spira, Rowan and CAAT relies upon productive dialogue and discussion between stakeholders and collaborative efforts to produce change. The scientific benefits of developing new methodologies have also been stressed. The focus of activity is always the respectful exchange of information between parties who may disagree on certain issues, but who are willing to work together to find mutually acceptable solutions. This policy differs profoundly from that pursued by many animal rights groups and defenders of the scientific status quo, who fear that such communication will result in a weakening of their position and unacceptable concessions. “Constructive negotiations are far more productive than ongoing confrontations,” as Spira often noted.
The policy of negotiation pursued by Spira, Rowan, the Hopkins Center and other individuals and groups throughout the world who have adopted a similar outlook has been greatly facilitated by adoption of the replacement, reduction, refinement (3Rs) definition of alternatives proposed by British researchers W.M.S. Russell and Rex Burch in their book The Principles of Humane Experimental Technique, published in 1959. Russell and Burch theorized that pain and distress in research animals inevitably result in inaccurate and misleading experimental data, therefore scientists and other laboratory personnel must eliminate animal suffering and the causes of suffering in order to produce reliable results (Russell and Burch, 1959). To that end, Russell and Burch proposed that scientists refine experimental procedures to eliminate causes of pain and distress, reduce the number of animals to the minimum required to achieve the experimental end, and replace animals whenever possible with either cell and tissue culture models (in vitro studies) or other methodologies. Russell and Burch’s 3Rs thus provide a blueprint for cooperation between scientists and animal protectionists, although they do not satisfy those who wish for immediate abolition of animal research or those who are averse to examining scientific policy and practice.
Motivation to Adopt
The cosmetics industry initially adopted the approach described above out of expediency - customers were demanding products that had not been tested on animals and both the media and the public were exerting a great deal of pressure on companies to develop alternative models of testing. Almost immediately many realized that the development of alternatives would benefit the industry in a number of ways. The biochemical assays and databases developed as alternatives would likely cost less and be more predictive of human response than the animal tests they were created to replace. Human cell and tissue culture in particular were regarded as highly promising methodologies which might prove quite useful, once preliminary research was able to identify certain biological endpoints which could be used to predict toxicity.
As historian John Parascandola has noted, “although pressure from the animal rights movement has played a key role in stimulating interest in alternatives to animal experimentation and testing, scientific and economic considerations have also figured prominently in these developments.” (Parascandola, 1991). Development of new methodologies is dependent on scientific advances and technical improvements in tissue culture techniques, for example, have greatly facilitated the development of alternatives just as molecular and cellular approaches have provided a method to explore early biological responses to chemical or physical agents and the role of these early effects in altered cellular structure and function (Sutter, 1995).
The Center for Alternatives to Animal Testing has been a unique resource in the United States because of its association with an academic institution. This affiliation has been crucial in establishing and maintaining CAAT’s reputation as a scientifically based organization attempting to balance the need to protect the health and safety of the public with the goal of reducing, refining and replacing animal use for research, education and testing. CAAT’s association with Johns Hopkins University, one of the premier research institutions in the world, has helped the organization attract scientists (traditionally distrustful of animal protection activity) willing to investigate the development of in vitro methods for product safety testing. CAAT has founded over 200 grant applications since 1981 and organized symposia, workshops and other forums to facilitate the exchange of information between researchers as well as between scientists, animal protectionists and the public (Zurlo & Goldberg, 1999).
In Vitro Testing and SAR: Two Models
(excerpted from Zurlo, Rudacille and Goldberg: Animals and Alternatives in Testing: History, Science and Ethics)
In vitro assays consist of three components--the biological model, the endpoint measurement, and the test protocol. The biological model is the system used for evaluation. The greater the ability of the biological model to represent the in vivo (whole animal) structure and function, the more valuable the data. An endpoint measurement is the yardstick used to predict toxicity (for example, cell death). The test protocol is the schedule of events defining the test; for example exposing liver cells to a test chemical for a certain period of time and measuring the defined endpoint at various times after rinsing the chemical from the dish of cells.
The neutral red assay is an example of an in vitro test designed to provide an indication of cell membrane integrity as an endpoint. In this test, cells are cultured in plastic petri dishes and treated with various concentrations of a test chemical. The neutral-red dye, which is added to the cell culture after rinsing out the test chemical, is accumulated and stored by cells. The amount of dye retained by the cells indicates the number of living cells in the dish. A general test like the neutral-red assay thus provides some indication of cellular responses to chemicals that can then be interpreted as an indication of acute toxicity.
Structure-Activity Relationships (SAR) provide another avenue for research. The hypothesis on which this concept is based states that the structure of a chemical inherently possesses all of the information necessary to predict its toxicity, including the manner in which both the parent chemicals and its metabolites will interact with the macromolecules of a cell. In SAR, biological effects are expressed in quantitative terms. A mathematical equation is prepared to correlate the toxin’s chemical properties with biologic effects. The relationship derived from the equation is used to make predictions about the toxicity of a chemical. Computers are used to establish this relationship.
Obstacles to Implementation
The transition from animal models to human cells and tissues and other methodologies has been difficult for a number of reasons. First, the search for “quick fix” solutions has been proven a costly error in both economic and public relations terms. The development of alternatives is bound up with the progress of science in developing a deeper understanding of fundamental biological processes. Rarely if ever can a truly predictive test be created without some understanding of the mechanism which creates the effect it seeks to measure. Unfortunately, many members of the public and some animal protection groups believe that the only obstacle standing in the way of immediate abolition of animal use and adoption of alternatives is the stubbornness and ill will of conservative scientists. Unfortunately this is not the case. The replacement of in vivo tests with more predictive in vitro assays is part of an on-going process of scientific discovery, which does not adhere to any particular timetable or agenda. Naturally, many people would like to have replacement alternatives for most endpoints right now, just as many would like to have an AIDS vaccine right now, but both are dependent on the work of individual scientists and research groups and the process of individual insight and creative collaboration which is one of the hallmarks of science.
Notwithstanding these facts, it is clear that the fear of “giving in” to animal protectionists does underlie a great deal of resistance to the 3Rs among many scientists. For example, although many members of the Society of Toxicology are vocal advocates of more predictive tests which are better able to assess human response to chemicals (Goodman, 1999), they vehemently support continued animal testing and reject the 3Rs, for fear that a less militant response will be perceived as supporting the claims of animal protectionists that current animal models are irremediably flawed and ought to be replaced. As Gerhard Zbinden pointed out in 1987, “on balance, one comes to the conclusion that the predictive value of animal tests is not perfect, but better than its reputation.” Broad conclusions about the inefficacy of animal testing immediately alienate scientists who are aware of the utility of many existing protocols in protecting human health and safety.
Some scientists also resent criticism of current practices, and believe that attempts at reform tacitly imply that existing methodologies are cruel and/or inhumane. “When we advocate the Three R’s, we imply that we, as scientists, are currently doing something wrong, that we are a bit ashamed of being forced by necessity to do so, and that we would stop if we could. We appear to defend research use of animals as a necessary evil.” (Burke, 1991) Nonetheless, the underlying message of numerous surveys seems to be that most Americans are willing to accept the use of animals in scientific research when their concerns about animal suffering are taken seriously by the scientific community and adequately addressed. A greater understanding and acceptance of public ambivalence over animal use and the realization that animal use must be justified on a case by case basis and not merely in general terms, might help reduce resistance among scientists to adoption of the 3Rs.
Validation
A final, but necessary, obstacle to the implementation of alternative methodologies has been the need to validate new tests. Validation is an important theoretical construct which has proven difficult to translate into practical terms. The generally accepted definition of validation is “the process by which the credibility of a candidate test is established for a specific purpose with reliability and reproducibility verified.” The key words in this definition are “reliability,” and “reproducibility.” In order to be accepted as a replacement for an animal model, a validated non-animal test must be shown to be easily implemented in a variety of laboratories, and able to produce consistent credible results with few false positives or negatives. In other words, companies and regulatory agencies must feel certain that the new test or tests will provide them with trustworthy data that they can use to assess hazard and risk. Although this appears on first glance to be a relatively simple endeavor, creating a process through which the reliability and reproducibility of new tests can be established has proven a complex and difficult task.
As early as 1990, John M. Frazier, Associate Director of the Johns Hopkins Center for Alternatives to Animal Testing, was asked by the OECD to prepare a report on “Scientific Criteria for Validation of In Vitro Toxicity Tests” (Frazier, 1990). Later that year, CAAT and the European Research Group for Alternatives to Animal Testing (ERGATT) conducted a workshop January 8-12, 1990 in Amden Switzerland. The report published following the meeting identified validation as an issue “of crucial significance.” Recognizing that “although most scientists have an inherent concept of what constitutes the validation of test procedures, the scientific basis and the necessary components of this process have never been fully described in a formal exposition,” participants at the workshop reiterated key elements of the Frazier monograph, identifying essential features of the validation process. Even at that early stage, many of the issues which were to hamstring future efforts to validate alternative tests were recognized as potential problems. The workshop’s first three recommendations in particular were only superficially addressed over the next six years in various validation studies, some of them quite ambitious and expensive.
1) The purpose of a validation study should be fully defined, particularly in relation to the level of assessment (toxic potential, toxic potency, hazard or risk) and in relation to the type of test required (screening, adjunct or replacement), the type of toxicity to be evaluated, and the chemical spectrum of interest.
2) Tests should only be considered for inclusion in validation studies, if the specific purposes for which they have been developed are well-defined and are consistent with the overall objectives of the validation study.
3) Tests must have been adequately developed, standardized and documented, and a need for them in relation to the availability of other tests must exist, before they should be considered eligible for validation. (Balls, Blaauboer, Brusick et al., 1990)
International Controversy
As academic toxicologists, regulatory officials and industry attempted to work out a process which could prove the efficacy and efficiency of newly developed tests, political pressures began to build for legislative bans on animal testing. In Europe the pressures were particularly intense and in 1993, the Council of Ministers to the European Communities passed a piece of legislation commonly known as the “Cosmetics Directive” which banned “the marketing in Europe of cosmetic products or ingredients tested on animals after January 1, 1998, unless alternative methods are insufficiently validated.” At around the same time, the European Union (EU) established the European Center for the Validation of Alternative Methodologies (ECVAM) in Ispra, Italy to facilitate the process of validation.
These developments in Europe were greeted with apprehension in the U.S. and Canada. Both regulatory officials and scientists in industry and academia in North America thought that the European ban was premature--an attempt to force science to conform to politics. Validation studies in progress were methodologically flawed, critics charged, because they failed to conform to the rigorous standards which were established as early as the Amden meeting. Pressured by the Cosmetics Directive and the rapidly looming deadline, companies and national governments labored to validate acceptable tests and failed -- partially because the validation studies were indeed flawed and partially because the science of in vitro toxicology was in its infancy and the first generation of tests proposed for validation were incapable of fulfilling the ambitious role in safety testing promised by their advocates. For five years, expensive validation projects in Europe produced profoundly ambiguous results. In the end, the Cosmetics Directive fell victim to GATT, the international trade agreement, and was never implemented. The ban on animal tests was ruled an example of a trade restriction that unfairly favored certain nations and so despite its strong support by EU nations, it could not be implemented.
The focus on validation then shifted to the U.S., where a more cautious, scientifically- based approach has prevailed. An ad hoc group of regulatory officials who called themselves IRAG, (the Interagency Regulatory Alternatives Group), had by 1995 been succeeded by ICCVAM (the Interagency Coordinating Committee on the Validation of Alternative Methods). While IRAG was composed of self-nominated members from only 3 federal agencies--the Food and Drug Administration (FDA), Environmental Protection Agency (EPA) and the Consumer Product Safety Commission (CPSC)--ICCVAM by federal mandate includes members from 13 regulatory and research agencies. Its goals are to encourage the development of improved testing methods that will generate data more useful for risk assessment; lead to the scientific evaluation/validation of new and revised test methods; increase the likelihood of acceptance of scientifically valid new and revised test methods; and encourage the refinement and reduction of animal use in testing, and the replacement of animals with non-animal methods and/or phylogenetically lower species, when scientifically feasible.
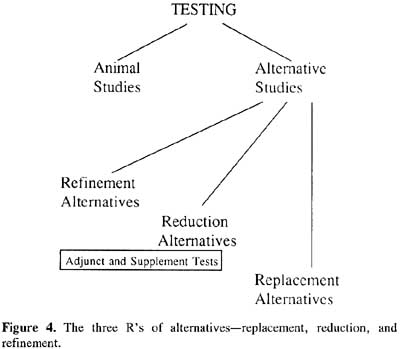 |
By late 1999, two methods had been evaluated by ICCVAM and subsequently accepted by the U.S. Food and Drug Administration, Environmental Protection Agency, Consumer Product Safety Commission, and Occupational Safety and Health Administration. The methods re the local lymph node assay for guinea pig hypersensitization and Corrositex, a biochemical assay. Both tests are replacements for the Draize test for skin irritation.
Current Challenges
On October 9, 1998 the Clinton Administration, the Environmental Defense Fund and the Chemical Manufacturers’ Association jointly announced a six-year program to test 2800 major industrial chemicals for their health and environmental effects. This announcement followed three major studies which indicated that most industrial chemicals produced in high volume in the U.S. lack even the most rudimentary information on potential health effects (EDF, 1997; CMA, 1998; EPA, 1998) In an attempt to remedy this situation, chemical manufacturers have agreed to participate in a “voluntary challenge program” to provide basic toxicity information, a Screening Information Data Set (SIDS) on their High Production Volume (produced in greater quantities than 1 million pounds) or HPV chemicals. EPA will then pursue additional testing of chemicals believed to persist in the environment and exhibit cumulative effects on the health of human beings, animals and the environment. This program is based on the OECD’s SIDS program initiated a decade earlier, although the timetable for data accumulation and reporting has been much accelerated in the U.S. program.
The HPV program, one aspect of the Chemical Right to Know Initiative announced by Vice-President Al Gore on Earth Day 1998, has come under attack by a number of animal protection organizations, particularly People for the Ethical Treatment of Animals (PETA), which has initiated a campaign attacking the HVP program in general and Vice President Gore in particular. On February 24, 1999 PETA ran a full-page ad in The New York Times denouncing Gore and his support of the HPV and RTK testing programs. “If you think Al Gore is an environmentalist, think again. First he was caught in a secret White House memo supporting commercial whaling. Now he’s pushing a government program that will kill millions of birds, fish, rabbits and other animals in useless and painful experiments,” the ad read. “It may sound like something to vote for but it’s really just bad news for the environment, the animals and public health. Animal tests are so unreliable that they can actually clear chemicals we already know to be harmful to humans. Or, when animals die, companies can claim the results don’t apply. Either way, government action will be delayed for years. Modern, reliable, non-animal tests are available but are being ignored.”
On June 17, 1999, the House Committee on Science, Subcommittee on Energy and Environment, held its first hearing on the HPV Chemical Testing Program. The hearing was held three months after Science Committee Chairman F. James Sensenbrenner sent a letter to EPA director Carol M. Browner, requesting the agency’s response to ten questions on the methodological and scientific assumptions underlying the program. In his opening statement Ken Calvert, chair of the House energy and environment subcommittee, expressed his dissatisfaction with the EPA and their planned strategy in the strongest terms, questioning both the scientific assumptions upon which the program is based (“production quantity does not necessarily entail risk...there are certainly more serious threats posed by lower production, higher toxicity chemicals. Shouldn’t we focus on them ?”) and the methodologies which will be used to assess toxicity (“Significant doubt exists about the validity of animal testing results when applied to humans,” he said. “I would like to hear about some of the humane alternatives to animal testing.”) Calvert concluded his remarks by noting that “it might be better to go back to the drawing board on the HPV program, spend a little bit more time and apply some well-considered sound science to design a better chemical testing program.”
Meanwhile, the Johns Hopkins Center for Alternatives to Animal Testing, and its partners at the Environmental Defense Fund, University of Pittsburgh, and Carnegie-Mellon University, have adopted a policy termed “TestSmart,” characterized as a humane and efficient approach to acquiring SIDS Data. “The immediate objective is to reduce the number of animals tested by incorporating in vitro and other alternatives into the SIDS battery of tests. Our long-term goal is to provide a more humane and efficient model for collecting data for hazard evaluation and safety assessment,” the program literature states. “In the long run, we believe appropriate molecular-based, mechanistic assays can be developed that will lead to a more accurate assessment of chemicals--and to a safer environment for the public.” CAAT held a TestSmart workshop on April 26-17, 1999 in Fairfax, VA. Participants included representatives of the Environmental Defense Fund, OECD, EPA, Humane Society of the United States, PETA, Physicians Committee for Responsible Medicine, National Institute of Environmental Health Sciences, Food and Drug Administration, ICCVAM, ECVAM and various academic researchers creating new testing methodologies, including the use of human cells in culture.
Workshop participants created a list of recommendations which included the following goals:
1) Reduction and refinement alternatives for acute toxicity testing should be incorporated, including the fixed dose procedure, the up-and-down procedure, the acute toxic class method and the limit test. (Each of these uses far fewer animals than traditional methods of acute toxicity testing and avoids death as an endpoint).
2) Existing in vitro tests for genetic toxicity should be employed immediately. These include the Ames-Salmonella and mouse lymphoma assays for bacterial and mammalian mutations and the Chinese Hamster Ovary cell assay for chromosomal aberrations.
3) In vitro tests evaluating sperm motility and sperm morphology should be used to screen for reproductive toxicity and SAR (structure-activity relationships) to establish chemical categories and to select specific chemicals within categories.
4) Protocols should be combined as much as possible to reduce animal numbers.
5) Promising in vitro tests should be evaluated as soon as possible and incorporated into the testing process. Potential candidates include the in vitro micronucleus assay for genetic toxicity, the FETAX, limb bud, and whole embryo culture assays for developmental toxicity and the basal cytotoxicity and neutral red uptake assays for acute toxicity.
The group also identified several promising areas worthy of additional research, including the use of organ cell cultures, human cells and tissue, and identification of mechanistic endpoints for toxicity screening. The full list of recommendations from this workshop and an explanation of the TESTSMART initiative are available on the ALTWEB site: http://altweb.jhsph.edu
TestSmart represents the continued effort to find common ground between scientists, regulatory officials and the activist community (environmental and animal protection). As in the earlier conflict over cosmetic testing, TestSmart offers a centrist approach, one which recognizes the validity of opposing viewpoints and seeks to create humane and efficient alternatives. Although the motivation of individuals adopting these principles may differ, a commitment to the goals of replacement, reduction and refinement offers a way to overcome the kind of protracted and pointless confrontation which serves neither human or animal well-being.
The TestSmart approach is being adopted by other groups and programs and may serve as a model for future efforts to achieve regulatory change. Those individuals and organizations who have adopted this model believe that in doing so they are following in the footsteps of Henry Spira, the activist whose efforts led to the founding of CAAT. “Rather than daydreaming about perfect solutions, activists need to push for the most rapid progress that can be realistically achieved,” Spira wrote in “Blueprint for Change,” first published in 1994 and reprinted in his 1997 guide, Strategies for Activists. Writing for activists who wish to promote corporate and government change, Spira (who died in September 1998) advised: “Keep building on previous achievements. Aim for initiatives that grow and proliferate, and become self-sustaining. It is an enterprise that develops a life of its own. Each action, each event is a step forward. And with each step forward, you can look further ahead.”
Literature Review/Bibliography
Ames B. (1989) “What are the major carcinogens in the etiology of cancer: six errors.” Important Advances in Oncology. (DeVita, Hellman and Rosenberg, eds.) Philadelphia: Lippincott.
Ames B. and Gold L. (1989) “Misconceptions regarding environmental pollution and cancer causation. Health Risks and the Press Perspectives on Media Coverage of Risk Assessment and Health.” Washington: The Media Institute.
Balls M., Blaauboer B., and Brusick D. et al. “Report and Recommendations of the CAAT/ERGATT Workshop on the Validation of Toxicity Test Procedures.” 1990. ATLA 18; Technical Report No. 3, The Johns Hopkins Center for Alternatives to Animal Testing.
Blum D. 1994. The Monkey Wars. New York and Oxford: Oxford University Press.
Bekoff M. 1998. Encyclopedia of Animal Rights and Animal Welfare. Westport CONN: Greenwood Press.
Burke R. 1991. Proceedings of the NIH OPRR/OACU Conference on Animal Care and Use: Policy Issues in the 1990s. (pp. 75-76). Bethesda MD: National Institutes of Health.
Francione G. 1996. Rain Without Thunder: The Ideology of the Animal Rights Movement. Philadelphia: Temple University Press.
Goldberg A.M. 1982-1998. Alternative Methods in Toxicology, Vol. 1-12. New York: Mary Ann Liebert.
Green S. and Bradlaw J. 1992. “Regulatory law and the use of in vitro methods for the assessment of various toxicities” in In Vitro Toxicity Testing. (John M. Frazier, ed). new York: Marcel Dekker.
Jasper J. and Nelkin D. 1992. The Animal Rights Crusade: The Growth of a Moral Protest. New York: The Free Press.
Lamb R. 1926. American Chamber of Horrors: The Truth About Food and Drugs. New York: Farrar and Rinehart.
Lederer S. 1987. “Controversy in America, 1880-1914” in Vivisection in Historical Perspective. (Nicolaas Rupke, ed). London and New York: Routledge.
Lederer S. 1995. Subjected to Science: Human Experimentation in America before the Second World War. Baltimore & London: Johns Hopkins University Press.
Loeper J. 1991. Crusade for Kindness: Henry Bergh and the ASPCA. New York: Atheneum.
Niven, Charles D. 1967. History of the Humane Movement. London: Johnson.
McCulley J. and Stephens T. 1993. “Draize Eye Testing Alternatives: A Perspective” in In Vitro Toxicology: Tenth Anniversary Symposium of CAAT (Alan M. Goldberg, ed). New York: Mary Ann Liebert.
Parascandola J. 1991. “Historical Perspectives on In Vitro Toxicology” in In Vitro Toxicology: Mechanisms and New Technology. (Alan M. Goldberg, ed). New York: Mary Ann Liebert.
Regan T. 1983. The Case for Animal Rights. Berkeley CA: University of California Press.
Regan T and Singer P. 1976. Animal Rights and Human Obligations. Englewood Cliffs NJ: Prentice Hall.
Rowan A. 1987. “Alternatives - A Kaleidoscope of Perspectives” in In Vitro Toxicology: Approaches to Validation. New York: Mary Ann Liebert.
Rowan A., Loew F., and Weer J. 1995. The Animal Rights Controversy: Protest, Process, and Public Policy. Boston: Tufts University School of Veterinary Medicine.
Rudacille D. 1992. “CAAT marks anniversary with scientific program, ceremony.” Newsletter of the Johns Hopkins Center for Alternatives to Animal Testing. 10 (1).
Rudacille D. 2000. The Scalpel and the Butterfly: The War Between Animal Research and Animal Protection. New York: Farrar, Straus & Giroux (in press).
Russell WMS and Burch RL. 1959. The Principles of Humane Experimental Technique. London: Metheun & Co.
Singer P. 1975. Animal Liberation. New York: New York Review of Books and Avon Books.
Singer P. 1998. Ethics into Action: Henry Spira and the Animal Rights Movement. Oxford: Rowan & Littlefield Publishers.
Sobels FH. 1977. “Some problems associated with the testing for environmental mutagens and a perspective for studies in comparative mutagenesis.” ARCH TOXICOL 46: 21-30.
Spira H. 1985. Winning with Archimedian Principles. ATLA 13 (2).
Sutter TR. 1995. Molecular and Cellular Approaches to Extrapolation for Risk Assessment. Environmental Health Perspectives: 103(4).
Turner J. 1980. Reckoning with the Beast: Animals, Pain and Humanity in the Victorian Mind. Baltimore & London: Johns Hopkins University Press.
Weil C & Scala R. (1971) “Study of Intra and Interlaboratory Variability in the Results of Rabbit Eye and Skin Irritation Tests.” TOXICOL APPL PHARMACOL 19: 276-360.
Zurlo J. and Goldberg A.M. 1999. “The Role of an Academic Center in Promoting Common Goals.” CAMBRIDGE QUARTERLY OF HEALTHCARE ETHICS. Cambridge: Cambridge University Press.
Zurlo J., Rudacille D., and Goldberg A.M. (1994). Animals and Alternatives in Testing: History, Science and Ethics. New York: Mary Ann Liebert.
Submitted by:
Deborah Rudacille
Baltimore, MD
USA
email:rudacill@clark.net
Information Date: 1999-10-01
Information Source: Deborah Rudacille
Search
Latest articles
Agriculture
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Air Pollution
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Biodiversity
- It is time for international mobilization against climate change
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
Desertification
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- UN Food Systems Summit Receives Over 1,200 Ideas to Help Meet Sustainable Development Goals
Endangered Species
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
- Coral Research in Palau offers a “Glimmer of Hope”
Energy
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Wildlife Preservation in Southeast Nova Scotia
Exhibits
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
- Coral Reefs
Forests
- NASA Satellites Reveal Major Shifts in Global Freshwater Updated June 2020
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Global Climate Change
- It is time for international mobilization against climate change
- It is time for international mobilization against climate change
Global Health
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- More than 400 schoolgirls, family and teachers rescued from Afghanistan by small coalition
Industry
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
Natural Disaster Relief
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
- Global Innovation Exchange Co-Created by Horizon International, USAID, Bill and Melinda Gates Foundation and Others
News and Special Reports
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- STOP ATTACKS ON HEALTH CARE IN UKRAINE
Oceans, Coral Reefs
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Pollution
- Zakaria Ouedraogo of Burkina Faso Produces Film “Nzoue Fiyen: Water Not Drinkable”
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Population
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
Public Health
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Rivers
- World Water Week: Healthy ecosystems essential to human health: from coronavirus to malnutrition Online session Wednesday 24 August 17:00-18:20
- Mangrove Action Project Collaborates to Restore and Preserve Mangrove Ecosystems
Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Toxic Chemicals
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Actions to Prevent Polluted Drinking Water in the United States
Transportation
- "Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures" Volume 2 Is Now Available
- Urbanization Provides Opportunities for Transition to a Green Economy, Says New Report
Waste Management
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution
Water and Sanitation
- Honouring the visionary behind India’s sanitation revolution
- Honouring the visionary behind India’s sanitation revolution

